
锦秋A-Level学院拥有完备的学员服务系统,专业的师资团队、服务团队、留学团队为学生G5申请环环把关,一路保驾护航。总而言之,一切都是有可能的,没有什么是遥不可及的,专业申请策略加上个人的努力,英国G5不是梦!
今天给同学们分享下A-Level考试中的原子分子的计算复习。
Part 1 The Mole
1Relative atomic mass
The relative atomic mass, Ar, of an elementis the average mass of the naturally occurring isotopes of the element relative to the mass of an atom of carbon-12.
The relative molecular mass, Mr, is the mass of a molecule relative to the mass of an atom of carbon-12, which is given the exact mass of 12.
Example: NH3 = 14 + 3x1 = 17.
The relative formula mass is used for compounds made up of ions.
2The mole and Avagadros number
A mole of substance is the amount of substance that has the same number of particles as there are in 12.00g of carbon-12. The particles may be atoms, molecules, ions or even electrons.
This number of particles is a constant known as Avagadros constant (L), and is approximately 6 x 1023mol-1.
The mass of one mole of a substance is often referred to as the molar mass (M). The units of molar mass are g mol-1.
To find the amount of substance present in a given mass, we must divide that mass by the molar mass of the substance.
For example - if we had 10g of CaCO3:
M is 40 + 12 + 3x16 = 100g.
So in 10g of calcium carbonate there is 10/100 mol = 0.1 mol CaCO3.
To find the mass of a given substance, we multiply the number of moles of the substance by the molar mass.
3Calculations of mass from stoichiometric equations
If we know the mass of a reactant, we can find the mass of a product in a chemical equation.
Consider the formation of ammonia from hydrogen and nitrogen.
2N2 (56g) + 3H2 (6g) → 4NH3 (34g)
Hence, for every 6g of hydrogen we make 34g of ammonia.
Part 2 Mass Spectrometry
1Determination of Ar and Mr
An instrument called a mass spectrometer is used to calculate relative atomic mass.
In a mass spectrometer, atoms are converted to positive ions; these are then deflected by electric and magnetic fields before being detected. The mass of the elements individual isotopes are found as well as their abundance.
Example of a mass spectrum is shown below:
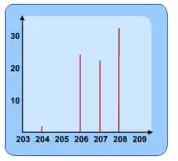
From this data the Relative Atomic Mass can be found.
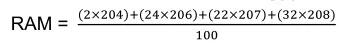
Part 3 Empirical and Molecular Formulae
1Definitions区分经验式和分子式的关系
The empirical formula of a compound shows the simplest whole-number ratio of the elements present.
The molecular formula shows the total number of atoms of each element present in a molecule of a compound.
Example:
hexane: C6H14
Empirical Formula: C3H7
Molecular Formula: C6H14
选择大于努力,家长在为孩子铺设留学道路的时候,摸爬滚打不如合理规划。选择一个专业的培训机构,家长和孩子都会少走很多弯路。新航道锦秋A-Level在为孩子规划课程时,首先会根据学生留学目的国的具体要求、学生自身的综合情况(如学习情况、性格、兴趣、未来打算等)综合衡量。
| 大学名称 | QS排名 |
|---|